Introduction to APIs – Active Pharmaceutical Ingredients
- Active Pharmaceutical Ingredients (APIs) are the chemical substances or compounds that are responsible for the therapeutic effect of a pharmaceutical drug. In simpler terms, APIs are the active components of a drug that provide its therapeutic effect. They are typically synthesized through chemical processes and undergo rigorous quality control and testing to ensure their safety and efficacy.
- APIs are the primary component of pharmaceutical drugs and are often combined with other ingredients such as excipients, preservatives, and fillers to form a final dosage form. These can include tablets, capsules, injections, topical creams, and more.
- APIs are regulated by health authorities in each country, and their production and use are subject to strict guidelines and regulations. These regulations ensure that APIs are of high quality, pure, and safe for human consumption.
Components of Active Pharmaceutical Ingredients (APIs)
- Chemical structures: APIs are complex organic or inorganic molecules that are synthesized through a series of chemical reactions. They have specific chemical structures that are responsible for their therapeutic activity.
- Excipients: Excipients are non-active ingredients that are added to APIs to form a final drug product. These can include binders, fillers, lubricants, and disintegrants that are necessary for the formulation of solid dosage forms such as tablets or capsules.
- Solvents: Solvents are used during the manufacturing process to dissolve and extract the API from raw materials. Common solvents used in API production include ethanol, methanol, and water.
- Catalysts: Catalysts are substances that speed up chemical reactions and are often used in API production to increase the yield or purity of the final product.
- Impurities: Impurities are substances that are present in APIs but are not the intended active ingredient. These can be generated during the synthesis of the API or can be introduced during the manufacturing process.
- Polymorphs: Polymorphs are different crystal forms of the same API compound that can have different physical and chemical properties. These can affect the solubility, stability, and bioavailability of the final drug product.
Production of Active Pharmaceutical Ingredients (APIs)
- Raw materials and starting materials: The production of APIs begins with the procurement of raw materials such as chemicals, solvents, and reagents. These materials are typically obtained from approved suppliers and must meet strict quality standards.
- Synthesis: The synthesis of the API involves a series of chemical reactions that are carried out in a controlled environment such as a laboratory or manufacturing plant. The reactions can vary depending on the specific compound being produced but typically involve the addition of reagents and solvents to create the desired chemical structure.
- Purification: Once the API has been synthesized, it must be purified to remove any impurities or byproducts. Purification can involve techniques such as crystallization, chromatography, and distillation.
- Formulation: Once the API has been purified, it can be formulated into a final dosage form such as a tablet, capsule, or injection. This involves combining the API with excipients, binders, and other ingredients to create a stable and effective drug product.
- Quality control: Throughout the production process, quality control measures are in place to ensure the safety, purity, and efficacy of the API. This can include testing of raw materials, in-process testing during synthesis and formulation, and final product testing to confirm that the API meets regulatory standards.
- Packaging and labeling: Once the API has been formulated into a final dosage form, it is packaged and labeled according to regulatory guidelines. This can include information on dosage, indications, contraindications, and potential side effects.
The Role of Quality Control in the Production of Active Pharmaceutical Ingredients (APIs)
Quality control plays a crucial role in the production of Active Pharmaceutical Ingredients (APIs) to ensure that the final product is safe, effective, and meets regulatory requirements.
- Raw material control: Quality control starts with the selection and testing of raw materials used in the production of APIs. Raw materials such as chemicals, solvents, and reagents must meet specific quality criteria and be sourced from approved suppliers. Quality control measures such as identity testing, purity testing, and microbiological testing are conducted to ensure that the raw materials are suitable for use in API production.
- In-process control: Quality control is carried out at various stages of API production to monitor the quality of the product. In-process testing includes checks on critical parameters such as temperature, pH, and pressure, as well as sampling and analysis of the product to check for impurities or deviations from the expected quality standards.
- Finished product testing: Quality control measures continue after the API has been synthesized and formulated into a final dosage form. Finished product testing involves a series of tests to ensure that the API meets regulatory requirements for purity, identity, potency, and stability. These tests are conducted on representative samples of the final product, and the results are compared against established specifications.
- Documentation and record-keeping: Quality control involves maintaining accurate records and documentation of all aspects of the production process, including raw material testing, in-process control, and finished product testing. This documentation serves as a record of the quality of the product and provides evidence of compliance with regulatory requirements.
- Continuous improvement: Quality control is an ongoing process that involves continuous monitoring and improvement. Data generated from quality control measures are analyzed to identify areas for improvement, and corrective actions are taken to prevent quality issues from recurring.
Conclusion
- Quality control plays a vital role in the production of APIs by ensuring that the final product meets strict quality standards and is safe and effective for use in pharmaceutical products. Quality control measures are in place throughout the production process, from the selection and testing of raw materials to finished product testing and continuous improvement thereby driving the Active Pharmaceutical Ingredients (API) Market
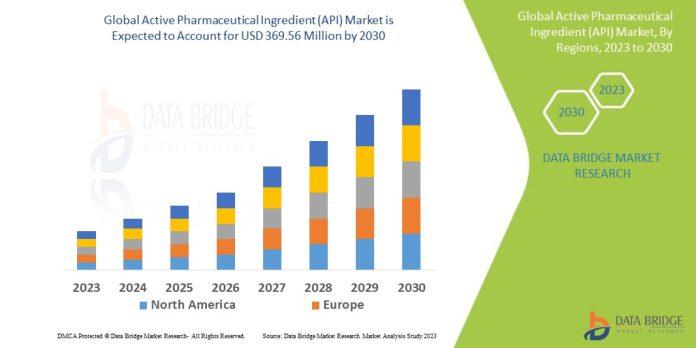
- Active Pharmaceutical Ingredients (API) Market Size
- The global market for active pharmaceutical ingredients (API) is anticipated to rise over the forecast period due to the rising demand for pharmaceutical medications. A number of market participants are trying to influence the production capacities of Asian nations to provide APIs to a number of other pharmaceutical companies. Additionally, manufacturers take precise measurements to determine each drug’s API strength.
- According to data bridge market research the active pharmaceutical ingredient (API) market is expected to reach USD 369.56 million by 2030, which is USD 223.3 million in 2022, and is expected to undergo a CAGR of 6.50% during the forecast period 2023 to 2030.
- For more insights on the active pharmaceutical ingredient (API) market visit https://www.databridgemarketresearch.com/reports/global-active-pharmaceutical-ingredient-api-market
- Also, visit https://nboxoffice.com/ for more articles