Biologics are drug products produced using a living organism. Biotherapeutics are often developed through the genetic engineering of living bacteria, plant, or animal cells. Today, biopharmaceuticals have played a critical role in advancing patient care through highly effective and targeted therapeutic interventions for numerous chronic and life-threatening diseases. However, due to their highly complex nature and large size, biopharmaceuticals need specialized techniques and approaches to manufacturing.
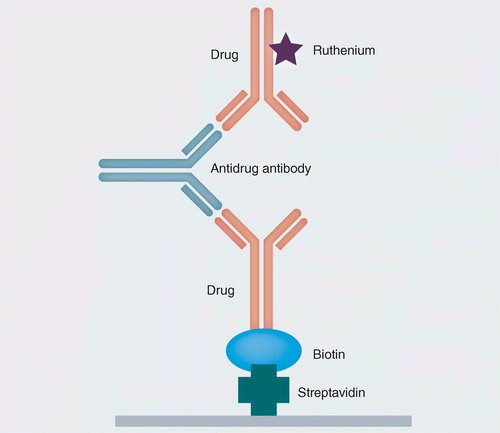
Due to their complex size and nature, biotherapeutics can elicit an immune response. These immune responses may impact the safety and efficacy profile of a pharmaceutical drug product. Hence, immunogenicity assessments become a crucial factor for every drug development initiative. Drug developers have numerous assay formats, such as MSD ELISA assay and flow cytometry analysis, to evaluate drug immunogenicity. The current article focuses on the innovations and advancements in immunogenicity assay development and the role it plays in accelerating drug development timelines.
Immunogenicity assay development
Immunogenicity assessments of biotherapeutics are mandatory for acquiring regulatory approvals. The US FDA has specific guidelines for immunogenicity assessments of biotherapeutic drug products. These recommendations include robust immunogenicity assay development for accurate detection and confirmation of anti-drug antibodies and subsequent NAb assay development. Besides, these recommendations focus on systemic data collection from clinical trials and standardized immunogenicity assessment across the entire life cycle of a biopharmaceutical drug product. Notably, regulatory guidance recommends not correlating animal immunogenicity testing results as an indicator of immune responses in human subjects. However, this experimental information can supplement subsequent preclinical and clinical toxicological studies.
As clinical trials are comparatively shorter, immunogenicity assessment results may not necessarily reflect clinical experience observed in the real world. Hence, continued immunogenicity assessment post approval is crucial for the long-term success of a biotherapy, as long-term treatment with biotherapeutics, for example, adalimumab for rheumatoid arthritis, showed that anti-drug antibodies negatively influenced the clinical response resulting in the discontinuation of the treatment.
On the other hand, clinical trials may miss out on detecting immune responses that may result in life-threatening outcomes. For example, post-administration of recombinant erythropoietin showed remarkably increased red cell aplasia in patients. This increase was observed post-approval of the biotherapeutic. Hence, continuous monitoring of drug immunogenicity after approval is critical. Drug developers and physicians should collaborate and focus their efforts on understanding laboratory and clinical observational data obtained from patients treated with a specific biotherapy to gain deeper insights into its immunogenicity profile. The US FDA recommends considering immunogenicity assessments in risk management plans and pharmacovigilance for all biotherapeutics.
Risk-based immunogenicity assessment followed for clinical trials is also generally proposed for post-approval monitoring of drug safety and efficacy. However, pharmacovigilance strategies should consider understanding risk factors due to sub-optimal anti-drug antibody detection in clinical studies. Hence, depending on the immunogenicity profile observed during clinical trials, the risk management strategy for unwanted immunogenicity should include pharmacovigilance, additional clinical studies, retrospective real-world analysis, and detailed information during prescription. Besides, drug developers should also consider situations when patients opt for a biosimilar, and hence, they must evaluate and assess the potential anti-drug antibody cross-reactivity in this specific patient population.