The “HPAPI and Cytotoxic Drugs Manufacturing Market (4th Edition), 2022-2035” report an extensive study of the current market landscape and future potential of the HPAPI and cytotoxic drug manufacturing market.
Key Inclusions
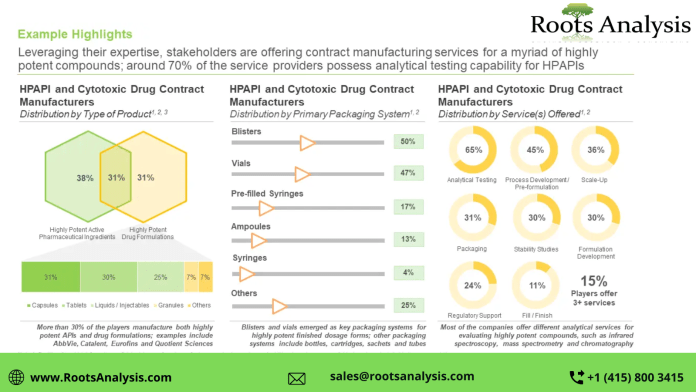
- A detailed assessment of the overall landscape of companies offering contract manufacturing services for HPAPI and cytotoxic drugs, along with information on several relevant parameters, such as year of establishment, company size (in terms of number of employees), location of headquarters, location of manufacturing facility, area of manufacturing facility (less than 10,000 sq. ft., 10,000 to 40,000 sq. ft., 40,001 to 80,000 sq. ft. and more than 80,000 sq. ft.), scale of operation (preclinical, clinical and commercial), type of product manufactured (HPAPIs and highly potent finished dosage forms), type of highly potent finished dosage form (capsules, tablets, liquids / injectables, granules and others), Occupational Exposure Limit (less than 0.1 µg/m3, 0.1 µg/m3 to 1 µg/m3 and more than 1 µg/m3), type of molecule manufactured (small molecules and biologics), type of primary packaging system (blisters, vials, prefilled syringes, ampoules, syringes and others), regulatory certifications / accreditations received and type of service(s) offered (analytical testing, process development / pre-formulation, scale-up, packaging, stability studies, formulation development, regulatory support and fill / finish).
- A detailed competitiveness analysis of HPAPI and cytotoxic drugs contract manufacturers, taking into consideration supplier strength (based on company size and their experience in this field) and service strength (based on scale of operation, number of highly potent finished dosage form, number of primary packaging system and number of service(s) offered).
- Elaborate profiles of prominent players (shortlisted based on a proprietary criterion) offering contract manufacturing services for HPAPI and cytotoxic drugs, across North America, Europe and Asia-Pacific. Each profile features a brief overview of the company, along with details related to its HPAPI and cytotoxic drug-related service portfolio, dedicated facilities, recent developments and an informed future outlook.
- An insightful analysis of the recent collaborations within the HPAPI and cytotoxic drug manufacturing industry, based on several relevant parameters, such as year of partnership, type of partnership (acquisitions, manufacturing agreements, research and development agreements, product development and commercialization agreements, technology licensing agreements and others), scale of operation (preclinical, clinical and commercial), type of product (HPAPI and high potent FDFs), most active players (in terms of number of deals inked) and regional distribution of partnership activity that have been undertaken in this domain, during the period 2014-2022.
- A detailed analysis of the recent expansions undertaken by several HPAPI and cytotoxic drug contract manufacturers, based on various relevant parameters, such as year of expansion, type of expansion (capacity expansions, facility expansions and new facility addition), company size (small, mid-sized, large and very large companies), location of headquarters, scale of operation (preclinical, clinical and commercial), type of product (HPAPI and high potent FDFs), location of expanded facility, area of expanded facility, amount invested in expansions, most active players (in terms of number of recent expansions) and geographical distribution.
- An estimate of the overall installed capacity for the manufacturing of HPAPIs, based on information reported by various industry stakeholders in the public domain. The analysis highlights the distribution of global installed capacity, based on company size (small, mid-sized and large), scale of operation (preclinical, clinical and commercial) and key geographical regions (North America, Europe, Asia-Pacific and Rest of the World).
- A regional capability assessment framework, which compares the HPAPI and cytotoxic drug manufacturing capabilities across key geographies, based on several parameters, such as the number of HPAPI and cytotoxic drug contract manufacturers, number of HPAPI and cytotoxic drug manufacturing facilities, number of facility expansions and installed HPAPI capacity in that particular geographical region.
- A detailed discussion on affiliated trends, key drivers and challenges, under a SWOT framework, which are likely to impact the industry’s evolution highlighting the relative effect of each SWOT parameter on the overall HPAPI and cytotoxic drug manufacturing market.
- A case study on companies offering manufacturing services for antibody drug conjugates (ADCs). The chapter also highlights the key components of ADCs and the key challenges associated with the manufacturing of these products. Further, the chapter presents a list of players that provide contract manufacturing services for ADCs.
The report also features the likely distribution of the current and forecasted opportunity across important market segments, mentioned below:
- Type of Product
- Highly Potent Active Pharmaceutical Ingredient
- Highly Potent Finished Dosage Form
- Company Size
- Small
- Mid-sized
- Large
- Very Large
- Scale of Operation
- Preclinical
- Clinical
- Commercial
- Type of Molecule
- Small Molecules
- Biologics
- Type of Highly Potent Finished Dosage Form
- Injectables
- Oral Solids
- Creams
- Others
- Key Geographical Regions
- North America (US, Canada and Mexico)
- Europe (UK, Italy, Germany, France, Spain and Rest of Europe)
- Asia-Pacific (China, India and Rest of Asia-Pacific)
- Rest of the World
Key Questions Answered
- Who are the key players engaged in offering contract manufacturing services for HPAPIs and cytotoxic drugs?
- What are the current opportunities within the HPAPI and cytotoxic drug market?
- What is the relative competitiveness of HPAPI and cytotoxic drug contract manufacturers?
- What types of partnership models are commonly adopted by stakeholders in this industry?
- What are the different types of expansion initiatives being undertaken by HPAPI and cytotoxic drug contract manufacturers?
- What are the key challenges faced by HPAPI and cytotoxic drug contract manufacturers?
- What are the key market trends and driving factors that are likely to impact this market?
- How is the revenue generation potential associated with HPAPI and cytotoxic drug manufacturing market likely to evolve in the coming years?
- How is the current and future market opportunity likely to be distributed across key segments?
To view more details on this report, click on the link
https://www.rootsanalysis.com/reports/view_document/hpapi-and-cytotoxic-drugs-manufacturing/299.html
You may also be interested in the following titles:
| Pharmaceutical Polymers / Medical Polymers Market |
| Gene Switch Market |
About Roots Analysis
Roots Analysis is a global leader in the pharma / biotech market research. Having worked with over 750 clients worldwide, including Fortune 500 companies, start-ups, academia, venture capitalists and strategic investors for more than a decade, we offer a highly analytical / data-driven perspective to a network of over 450,000 senior industry stakeholders looking for credible market insights.
Contact:
Ben Johnson
+1 (415) 800 3415